CTMS Reports and Exports
Run standard reports within one study, across studies, or across sponsors. Save report customizations to save time for other team members. Export study-specific views in common formats for further manipulation.
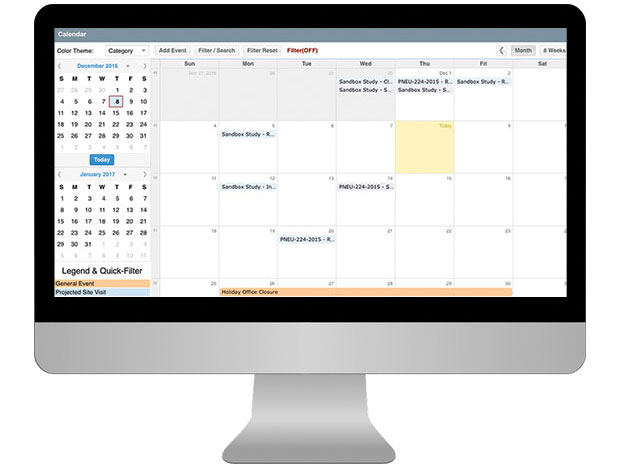
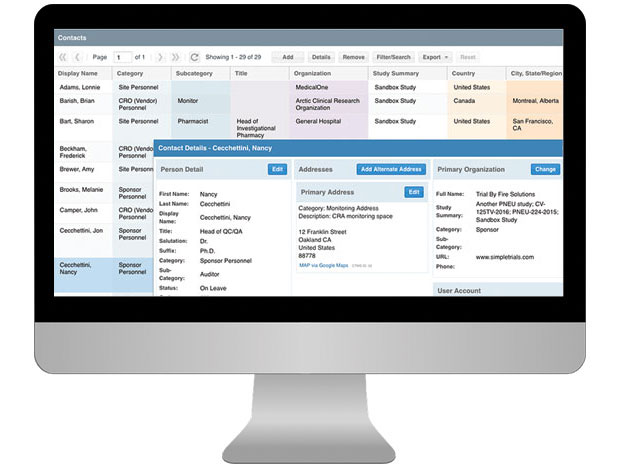
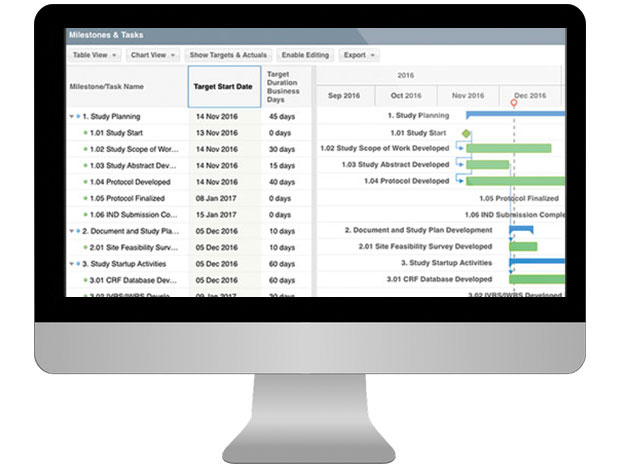
- Standard CTMS reports include rosters, study planning, calendar tracking, documents, study subjects and site contracts and payments.
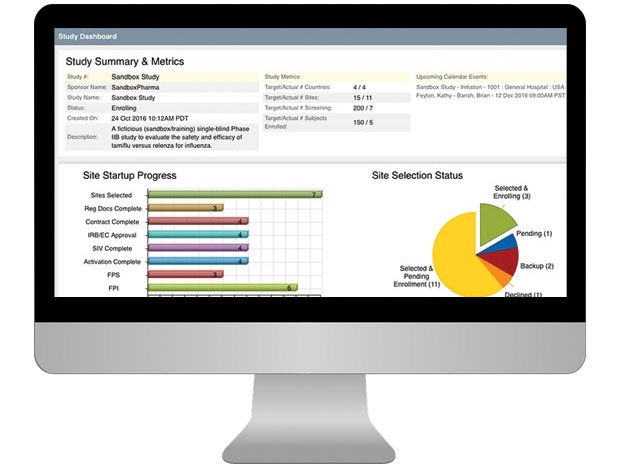
- Review startup tracking and milestones to assess efforts across studies and oversee resources needed
- View calendar visit schedules across teams and studies
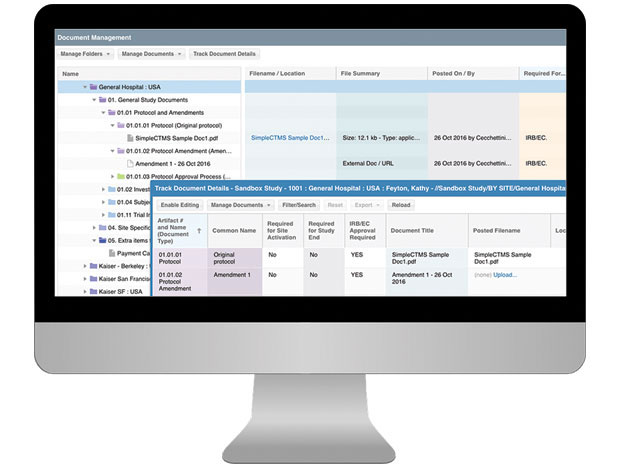
- Run document reports to highlight priority documents for study startup, draft documents to finalize, items for EC submission and items to have available for study closure
- Oversee recruitment and enrollment efforts within across studies to focus on screen failure reasons or subject retention hurdles
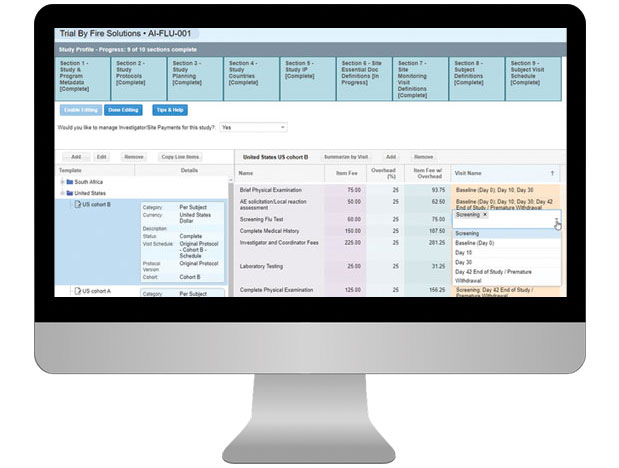
- Manage study finances by sponsor, study and overall while tracking status of invoice and payment efforts